Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
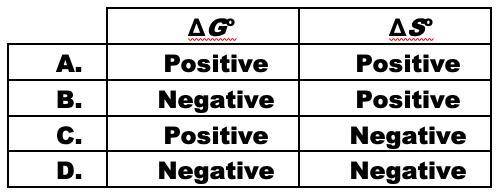
When ammonium nitrate dissolves in water, the solution becomes cold.
NH4NO3(s) <-> NH4+(aq)...
Questions


Physics, 25.11.2021 05:50

Social Studies, 25.11.2021 05:50


Mathematics, 25.11.2021 05:50


Computers and Technology, 25.11.2021 05:50


Mathematics, 25.11.2021 05:50

Mathematics, 25.11.2021 05:50


Computers and Technology, 25.11.2021 05:50


Chemistry, 25.11.2021 05:50

English, 25.11.2021 05:50

Physics, 25.11.2021 05:50

Chemistry, 25.11.2021 05:50