
Chemistry, 10.05.2021 23:40 evandlubbep6bsvu
PLEASE HELP
A group of students designs a simple system that could be used to generate heat. The students use the compound
sodium acetate (NaC, H,O,) for the experiment. They dissolve a specific amount of NaC, H30, in 100 grams (9) of
water placed in an insulated cup. They measure the initial temperature of the water in degrees Celsius (°C) and the
maximum temperature reached by the mixture of water and NaC, H,O2. The diagram shows their experimental
setup.
Thermometer
-Insulated cup
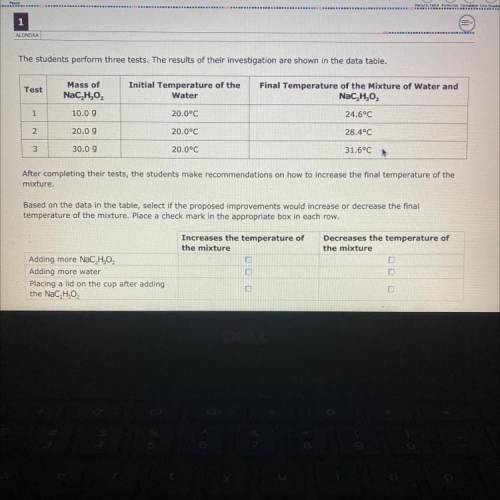
The students perform three tests. The results of their investigation are shown in the data table.
Test
Mass of
NaC, H,O2
Initial Temperature of the
Water
Final Temperature of the Mixture of Water and
NaC H2O2
1
10.09
20.0°C
24.6°C
2.
20.09
20.0°C
28.4°C
3
30.09
20.0°C
31.6°C

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
PLEASE HELP
A group of students designs a simple system that could be used to generate heat. The s...
Questions

Mathematics, 01.07.2019 23:30


Mathematics, 01.07.2019 23:30

Mathematics, 01.07.2019 23:30

Chemistry, 01.07.2019 23:30

History, 01.07.2019 23:30

Biology, 01.07.2019 23:30

History, 01.07.2019 23:30

Biology, 01.07.2019 23:30

Biology, 01.07.2019 23:30





Social Studies, 01.07.2019 23:30







