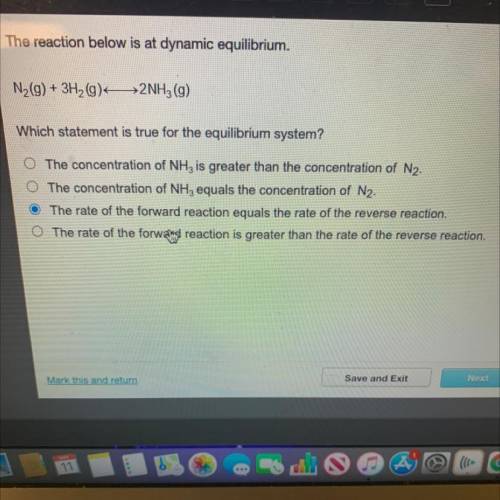
N(9) + 3H2(9) 2NH3 (9)
Which statement is true for the equilibrium system?
The concentration...

N(9) + 3H2(9) 2NH3 (9)
Which statement is true for the equilibrium system?
The concentration of NH, is greater than the concentration of N2.
O The concentration of NH3 equals the concentration of N2.
The rate of the forward reaction equals the rate of the reverse reaction.
O The rate of the forwald reaction is greater than the rate of the reverse reaction.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1


Chemistry, 22.06.2019 09:30
The chart shows the bid provided by four contractors to complete a job. which contractor is the most cost-effective?
Answers: 3
You know the right answer?
Questions




History, 20.05.2020 00:57

French, 20.05.2020 00:57

Biology, 20.05.2020 00:57




Mathematics, 20.05.2020 00:57

Mathematics, 20.05.2020 00:57

German, 20.05.2020 00:57

Biology, 20.05.2020 00:57


English, 20.05.2020 00:57

History, 20.05.2020 00:57

History, 20.05.2020 00:57



Mathematics, 20.05.2020 00:57



