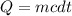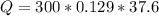Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
How much energy is required to raise the temperature of a 300.0
gram block of lead from 22.3°C to 5...
Questions

Mathematics, 19.11.2020 14:00

English, 19.11.2020 14:00

Physics, 19.11.2020 14:00

Biology, 19.11.2020 14:00



Chemistry, 19.11.2020 14:00

Biology, 19.11.2020 14:00


History, 19.11.2020 14:00

English, 19.11.2020 14:00

English, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00


Chemistry, 19.11.2020 14:00

Chemistry, 19.11.2020 14:00

Computers and Technology, 19.11.2020 14:00

Mathematics, 19.11.2020 14:00

History, 19.11.2020 14:00

English, 19.11.2020 14:00