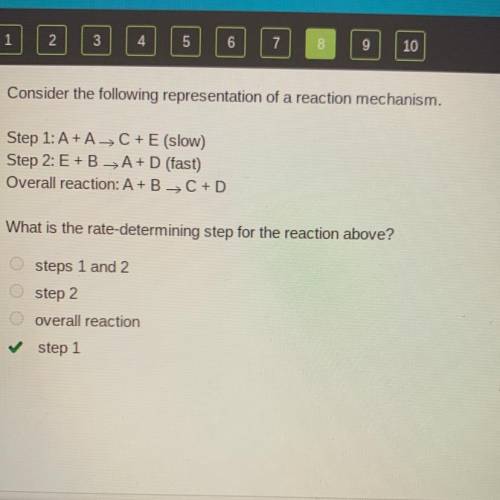
Consider the following representation of a reaction mechanism.
Step 1: A+ A-> C+E (slow)
S...

Chemistry, 11.05.2021 19:40 powella033
Consider the following representation of a reaction mechanism.
Step 1: A+ A-> C+E (slow)
Step 2: E+BA+D (fast)
Overall reaction: A+B C + D
What is the rate-determining step for the reaction above?
steps 1 and 2
step 2
overall reaction
step 1
To help others in the future

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
Questions







Computers and Technology, 21.11.2019 00:31
















