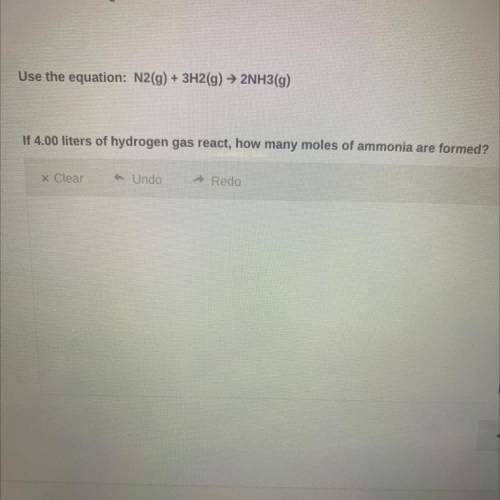
Use the equation:
N2(g) + 3H2(g) → 2NH3(g)
If 4.00 liters of hydrogen gas react, how ma...


Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3


Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

You know the right answer?
Questions

Chemistry, 22.02.2021 01:00

Mathematics, 22.02.2021 01:00


Mathematics, 22.02.2021 01:00


History, 22.02.2021 01:00

Mathematics, 22.02.2021 01:00



Mathematics, 22.02.2021 01:00

Mathematics, 22.02.2021 01:00

History, 22.02.2021 01:00

Mathematics, 22.02.2021 01:00







Mathematics, 22.02.2021 01:00