X-29: 28.976 amu; 4.68%

Chemistry, 11.05.2021 23:10 andrewbigbrains8740
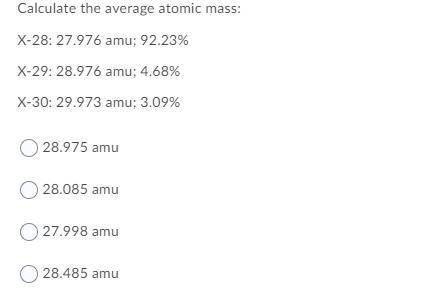
Calculate the average atomic mass:
X-28: 27.976 amu; 92.23%
X-29: 28.976 amu; 4.68%
X-30: 29.973 amu; 3.09%

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 2

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 14:30
Is a pencil falling to the floor anon contact force, a force, or a contact force
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
Calculate the average atomic mass:
X-28: 27.976 amu; 92.23%
X-29: 28.976 amu; 4.68%
X-29: 28.976 amu; 4.68%
Questions


Health, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30




Biology, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30

Biology, 18.03.2021 01:30

Mathematics, 18.03.2021 01:30



Biology, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

Social Studies, 18.03.2021 01:30


Mathematics, 18.03.2021 01:30

Health, 18.03.2021 01:30



