
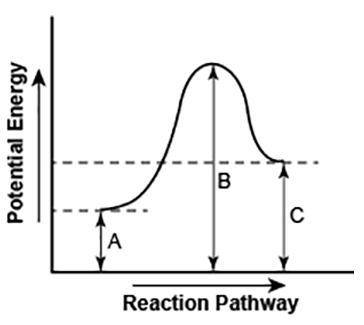
The diagram shows the potential energy changes for a reaction pathway. (8 points)
Part 1: Describe how you can determine the total change in enthalpy and activation energy from the diagram, and if each is positive or negative.
Part 2: Describe how the curve will look if the reaction was exothermic. Be sure to mention changes in the potential energies of the reactants and products and the sign changes of the enthalpy.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 19:50
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway. (8 points)
Part 1: Describe...
Questions



English, 10.06.2021 17:20




Mathematics, 10.06.2021 17:20


Social Studies, 10.06.2021 17:20

English, 10.06.2021 17:20


Mathematics, 10.06.2021 17:20




Mathematics, 10.06.2021 17:20

Mathematics, 10.06.2021 17:20



Mathematics, 10.06.2021 17:20



