
Chemistry, 12.05.2021 01:00 georgesarkes12
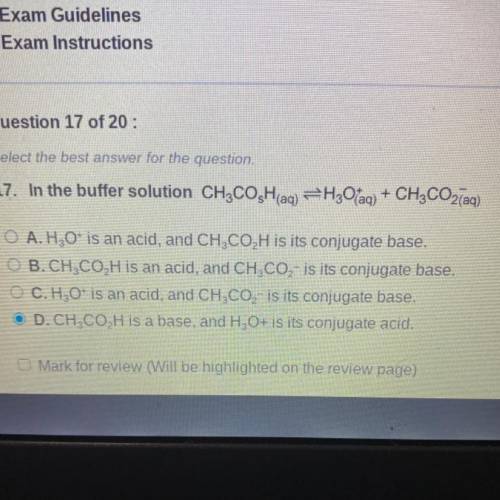
In the buffer solution CH3COsH(aq) -> H3O+(aq) + CH3CO2-(aq)
A. H3O+ is an acid, and CH3CO2H is its conjugate base.
B. CH3CO2H is an acid, and CH3CO2- is its conjugate base.
C. H3O+ is an acid, and CH3CO2- is its conjugate base.
D. CH3CO2H is a base, and H3O+ is its conjugate acid

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3


Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2
You know the right answer?
In the buffer solution CH3COsH(aq) -> H3O+(aq) + CH3CO2-(aq)
A. H3O+ is an acid, and CH3CO2H i...
Questions

English, 02.06.2021 21:20

History, 02.06.2021 21:20

Mathematics, 02.06.2021 21:20






Mathematics, 02.06.2021 21:20


Mathematics, 02.06.2021 21:20

Mathematics, 02.06.2021 21:20


Mathematics, 02.06.2021 21:20

Mathematics, 02.06.2021 21:20

Mathematics, 02.06.2021 21:20




Medicine, 02.06.2021 21:20




