
Chemistry, 12.05.2021 14:00 bg988763p7cl2d
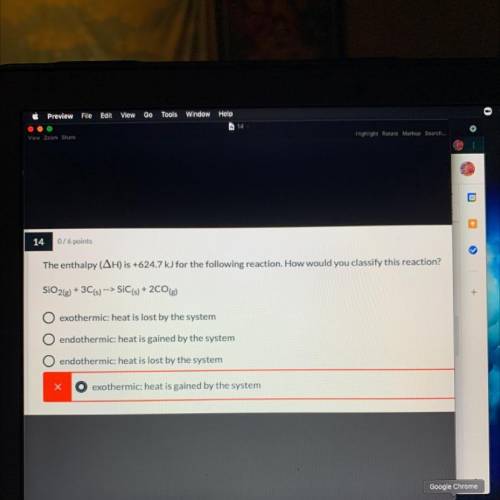
The enthalpy (AH) is +624.7 kJ for the following reaction. How would you classify this reaction? (Explain)
SiO2(g) + 3C(s) --> SIC(s) +2CO(g)
A. exothermic: heat is lost by the system
B. endothermic: heat is gained by the system
C. endothermic: heat is lost by the system
D. exothermic: heat is gained by the system

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
The enthalpy (AH) is +624.7 kJ for the following reaction. How would you classify this reaction? (Ex...
Questions




History, 25.09.2019 17:30

Mathematics, 25.09.2019 17:40


Mathematics, 25.09.2019 17:40



Mathematics, 25.09.2019 17:40

Physics, 25.09.2019 17:40



History, 25.09.2019 17:40

Mathematics, 25.09.2019 17:40

Mathematics, 25.09.2019 17:40


Social Studies, 25.09.2019 17:40

History, 25.09.2019 17:40

Social Studies, 25.09.2019 17:40

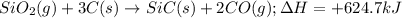 is classified as endothermic: heat is gained by the system.
is classified as endothermic: heat is gained by the system.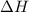 is positive which means heat is gained by the system.
is positive which means heat is gained by the system.

