
Chemistry, 12.05.2021 14:00 joelpimentel
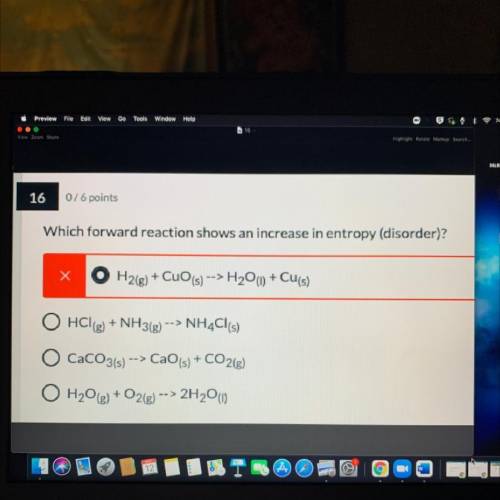
Which forward reaction shows an increase in entropy (disorder)?explain
A. H2(g) + CuO (s) --> H2O(l) + Cu(s)
B. HCl(g) + NH3(g) --> NH4Cl(s)
C. CaCO3(s) -> CaO(s) + CO2g)
D. H2O(g) + O2(g) --> 2H2O)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

You know the right answer?
Which forward reaction shows an increase in entropy (disorder)?explain
A. H2(g) + CuO (s) --> H2...
Questions

Mathematics, 20.02.2021 03:30

Mathematics, 20.02.2021 03:30


Mathematics, 20.02.2021 03:30


Mathematics, 20.02.2021 03:30

Mathematics, 20.02.2021 03:30

Chemistry, 20.02.2021 03:30


Mathematics, 20.02.2021 03:30


Mathematics, 20.02.2021 03:30


Mathematics, 20.02.2021 03:30


Physics, 20.02.2021 03:30

Mathematics, 20.02.2021 03:30

Mathematics, 20.02.2021 03:30


Mathematics, 20.02.2021 03:30



