
Chemistry, 12.05.2021 16:00 yasminothman02
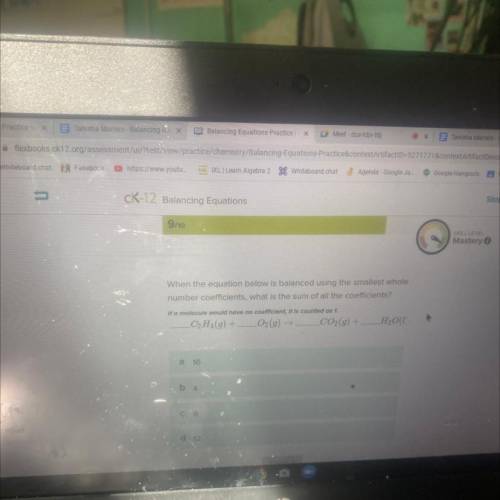
When the equation below is balanced using the smallest whole number coefficients, what is the sum of all the coefficients?
C2H4(g) + LO2(g) → CO2(g) +
H₂O1

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3
You know the right answer?
When the equation below is balanced using the smallest whole number coefficients, what is the sum of...
Questions

History, 21.10.2020 20:01

Geography, 21.10.2020 20:01

History, 21.10.2020 20:01

Advanced Placement (AP), 21.10.2020 20:01



Mathematics, 21.10.2020 20:01

Mathematics, 21.10.2020 20:01


Mathematics, 21.10.2020 20:01





Mathematics, 21.10.2020 20:01


Mathematics, 21.10.2020 20:01





