
Chemistry, 12.05.2021 16:40 cherishofomah04
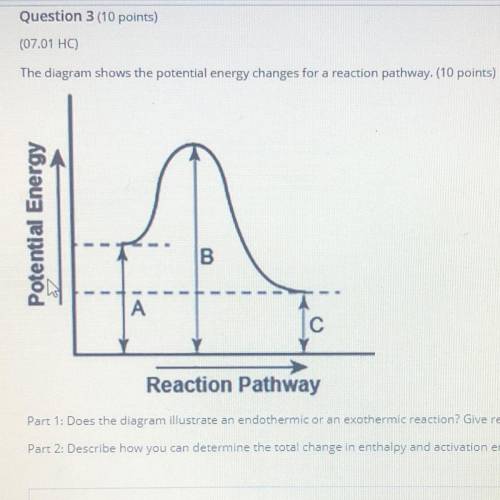
The diagram shows the potential energy changes for a reaction pathway. (10 points)
Potential Energy
B
А
Reaction Pathway
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer.
Part 2: Describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 21:30
Aphysical reaction is a process in which one or more reactants change into one or more products with different properties. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

Chemistry, 23.06.2019 01:30
Use the periodic table to determine how many grams of oxygen would be required to react completely with 859.0 g c2h2
Answers: 3
You know the right answer?
The diagram shows the potential energy changes for a reaction pathway. (10 points)
Potential Energy...
Questions


Spanish, 02.06.2021 19:50




English, 02.06.2021 19:50

English, 02.06.2021 19:50

Health, 02.06.2021 19:50

Mathematics, 02.06.2021 19:50


Spanish, 02.06.2021 19:50

Mathematics, 02.06.2021 19:50



Mathematics, 02.06.2021 20:00



Mathematics, 02.06.2021 20:00


Mathematics, 02.06.2021 20:00



