
Chemistry, 12.05.2021 17:40 mariahbugg7
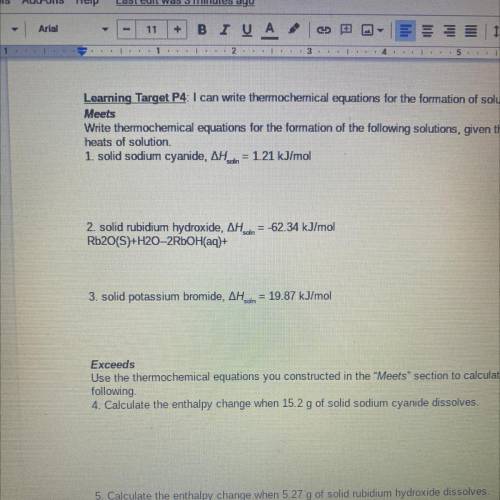
Write thermochemical equations for the formation of the following solutions, given their molar
heats of solution.
1. solid sodium cyanide, AH. din = 1.21 kJ/mol
2. solid rubidium hydroxide, AH dn = -62.34 kJ/mol
Rb2O(S)+H20-2RbOH(aq)+
I
3. solid potassium bromide, AH..in = 19.87 kJ/mol
Exceeds
Use the thermochemical equations you constructed in the "Meets" section to calculate the

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 07:50
Many reactions take place in aqueous solution. when potential reactants are mixed, a reaction will occur if there is some driving force that favors the formation of products. it is often convenient to categorize reactions in terms of these driving forces: precipitate formation, in which an insoluble solid is formed, weak electrolyte formation, as in a neutralization reaction involving water, or transfer of electrons, as in a redox reaction. these reactions can be represented by full molecular equations, which contain all species in the reaction mixture, or by net ionic equations, which show only the species that actually undergo a change. the latter does not contain the spectator ions, which do not undergo a net change or do not take part in the reaction. part a when the following two solutions are mixed: k2co3(aq)+fe(no3)3(aq) the mixture contains the ions listed below. sort these species into spectator ions and ions that react. drag the appropriate items to their respective bins. view available hint(s) spectator ions ions that react part b what is the correct net ionic equation, including all coefficients, charges, and phases, for the following set of reactants? assume that the contribution of protons from h2so4 is near 100 %.ba(oh)2(aq)+h2so4(aq)→ express your answer as a chemical equation. view available hint(s) nothing provide feedback
Answers: 3


Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2
You know the right answer?
Write thermochemical equations for the formation of the following solutions, given their molar
heat...
Questions

Social Studies, 20.10.2019 12:30



Mathematics, 20.10.2019 12:30



Social Studies, 20.10.2019 12:30

Mathematics, 20.10.2019 12:30

Mathematics, 20.10.2019 12:30

English, 20.10.2019 12:30

Biology, 20.10.2019 12:30

Social Studies, 20.10.2019 12:30



Mathematics, 20.10.2019 12:30



Geography, 20.10.2019 12:30

Chemistry, 20.10.2019 12:30

History, 20.10.2019 12:30



