Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1

Chemistry, 23.06.2019 03:00
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2
You know the right answer?
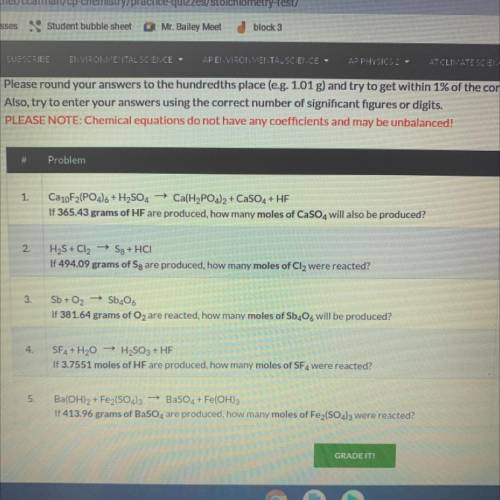
Ca10F2(PO4)6 + H2SO4 + Ca(H2PO4)2 + CaSO4 + HF
If 365.43 grams of HF are produced, how many moles o...
Questions

Mathematics, 19.11.2020 23:30


Mathematics, 19.11.2020 23:30



Mathematics, 19.11.2020 23:30

Advanced Placement (AP), 19.11.2020 23:30


Arts, 19.11.2020 23:30

Chemistry, 19.11.2020 23:30


Engineering, 19.11.2020 23:30

Mathematics, 19.11.2020 23:30

English, 19.11.2020 23:30

Mathematics, 19.11.2020 23:30

Health, 19.11.2020 23:30


Engineering, 19.11.2020 23:30

Mathematics, 19.11.2020 23:30

Mathematics, 19.11.2020 23:30