
Chemistry, 13.05.2021 01:00 jasonr182017
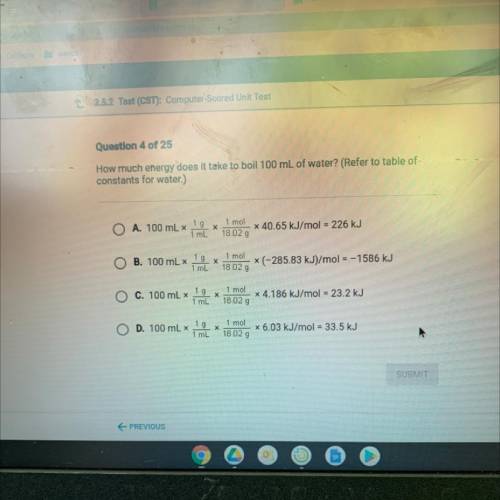
How much energy does it take to boil 100 mL of water? (Refer to table of
constants for water.)
A. 100 mL x
1 g
х
1 mL
1 mol
18.02 g
x 40.65 kJ/mol = 226 kJ
B. 100 mL x
1 g
1 mL
X
1 mol
18.02 g
(-285.83 kJ)/mol = –1586 kJ
C. 100 mL x
1 g
X
1 mol
18.02 g
x 4.186 kJ/mol = 23.2 kJ
1 mL
1 g
D. 100 mL x
1 mL
1 mol
x
18 02 g
x 6.03 kJ/mol = 33.5 kJ

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1
You know the right answer?
How much energy does it take to boil 100 mL of water? (Refer to table of
constants for water.)
Questions


Physics, 12.10.2019 08:50

Social Studies, 12.10.2019 08:50


Social Studies, 12.10.2019 08:50

Computers and Technology, 12.10.2019 08:50



Social Studies, 12.10.2019 08:50

Social Studies, 12.10.2019 08:50

Mathematics, 12.10.2019 08:50

English, 12.10.2019 08:50

Mathematics, 12.10.2019 08:50

Biology, 12.10.2019 08:50

Spanish, 12.10.2019 08:50

Biology, 12.10.2019 08:50

Mathematics, 12.10.2019 08:50

Mathematics, 12.10.2019 08:50


Biology, 12.10.2019 08:50



