
Chemistry, 13.05.2021 04:00 catherinesquitieri
An atom has the following electron configuration 1s2s2sp63s23p64s2 How many valance electrons does this atom have?
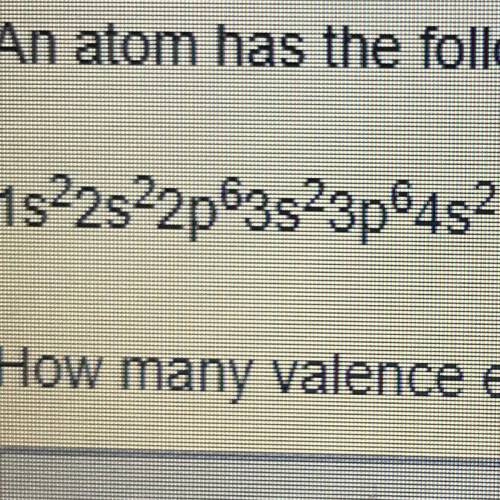

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 22.06.2019 03:00
In the 1800s, one of the statements in john dalton's atomic theory was that atoms are indivisible. later experimental evidence led to the discovery of subatomic particles such as neutrons, electrons, and protons. what happened to the indivisible atom part of dalton's atomic theory, and why?
Answers: 3

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
An atom has the following electron configuration 1s2s2sp63s23p64s2 How many valance electrons does t...
Questions

Physics, 19.03.2020 23:29


Engineering, 19.03.2020 23:29

Mathematics, 19.03.2020 23:29







Business, 19.03.2020 23:29

Mathematics, 19.03.2020 23:29


Law, 19.03.2020 23:29






Chemistry, 19.03.2020 23:29



