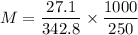Chemistry, 13.05.2021 05:30 PastelHibiscus
A solution was prepared by dissolving 27.1g of sucrose (C12H22O11) in 250 g of water. Find the molality of the solution(molar mass of C12H22O11 is 342.8)
-.108 m
-108 m
-317 m
-.317 m

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2
You know the right answer?
A solution was prepared by dissolving 27.1g of sucrose (C12H22O11) in 250 g of water. Find the molal...
Questions


Mathematics, 27.08.2021 06:10

Chemistry, 27.08.2021 06:10



Mathematics, 27.08.2021 06:10

English, 27.08.2021 06:10





Mathematics, 27.08.2021 06:10

Biology, 27.08.2021 06:10

English, 27.08.2021 06:10

Mathematics, 27.08.2021 06:10

Mathematics, 27.08.2021 06:10

Biology, 27.08.2021 06:10

Biology, 27.08.2021 06:10

English, 27.08.2021 06:10

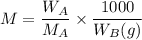
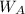 = mass of the solute = 27.1 g
= mass of the solute = 27.1 g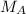 = molar mass of the solute = 342.8
= molar mass of the solute = 342.8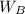 = mass of the given solvent = 250 g
= mass of the given solvent = 250 g