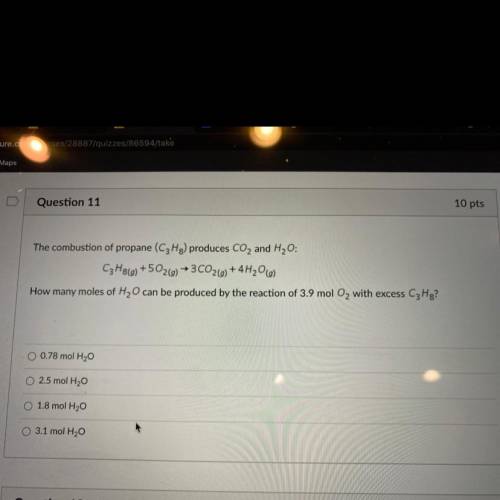
The combustion of propane (C3Hg) produces CO2 and H2O:
C3H8() +502(g) → 3CO2(g) + 4H20(g)
How...

Chemistry, 13.05.2021 06:20 supermansabeast
The combustion of propane (C3Hg) produces CO2 and H2O:
C3H8() +502(g) → 3CO2(g) + 4H20(g)
How many moles of H2O can be produced by the reaction of 3.9 mol O2 with excess C3H8?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 22.06.2019 02:20
6. what does the symbol ah stand for? o one calorie given off by a reaction the specific heat of a substance the heat capacity of a substance the heat of reaction for a chemical reaction
Answers: 1

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

You know the right answer?
Questions




Mathematics, 06.04.2020 09:56

Spanish, 06.04.2020 09:56



Mathematics, 06.04.2020 09:56

Social Studies, 06.04.2020 09:56





History, 06.04.2020 09:57


Business, 06.04.2020 09:57

Mathematics, 06.04.2020 09:57

History, 06.04.2020 09:57

Social Studies, 06.04.2020 09:57



