Answers: 3
Another question on Chemistry



Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
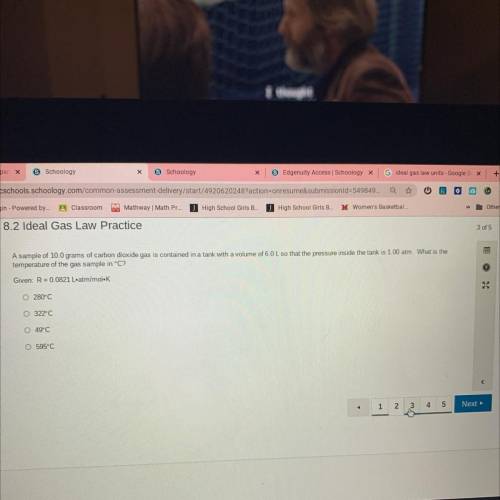
A sample of 10.0 grams of carbon dioxide gas is contained in a tank with a volume of 6.0 L so that t...
Questions




English, 05.05.2020 06:41

Mathematics, 05.05.2020 06:41

Mathematics, 05.05.2020 06:41




Mathematics, 05.05.2020 06:41

Mathematics, 05.05.2020 06:41

English, 05.05.2020 06:41

Chemistry, 05.05.2020 06:41




Mathematics, 05.05.2020 06:41

Arts, 05.05.2020 06:41

Mathematics, 05.05.2020 06:41