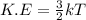Chemistry, 13.05.2021 20:00 osuigwewhitney487
PLEASE HELP
The picture shows two containers filled with a gas, both initially at room temperature.
Which statement is correct? (5 points)
1) The average kinetic energy of the gas particles is greater in container A because its particles move faster.
2)The average kinetic energy of the gas particles is greater in container Bbecause it has a lower temperature.
3) The gas particles in both containers have the same average kinetic energy
because they have the same volume.
4)The gas particles in both containers have the same average kinetic energy
because they have equal number of particles.


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1


Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
PLEASE HELP
The picture shows two containers filled with a gas, both initially at room temperature....
Questions


Mathematics, 07.05.2021 15:30


Mathematics, 07.05.2021 15:30



Chemistry, 07.05.2021 15:30



Mathematics, 07.05.2021 15:30

Mathematics, 07.05.2021 15:30


Mathematics, 07.05.2021 15:30

Biology, 07.05.2021 15:30

Mathematics, 07.05.2021 15:30


Mathematics, 07.05.2021 15:30