
Chemistry, 13.05.2021 20:10 sondrascott7351
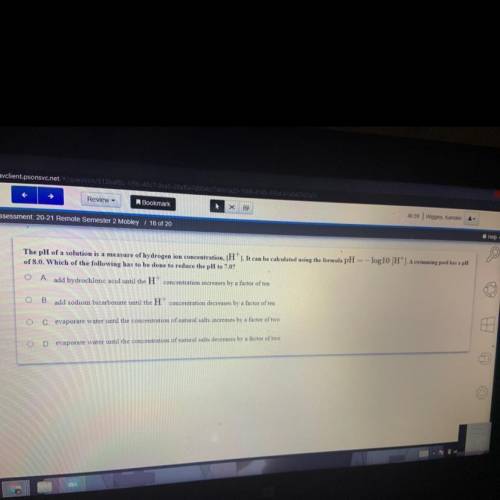
The pH of a solution is a measure of hydrogen ion concentration, (H'). It can be calculated using the formula pH = -log10 (H'). A swimming pool has a pH of 8.0. Which of the following has to be done to reduce the pH to 7.0?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:50
An aqueous solution of potassium hydroxide is standardized by titration with a 0.194 m solution of hydrobromic acid. if 25.2 ml of base are required to neutralize 24.2 ml of the acid, what is the molarity of the potassium hydroxide solution? m potassium hydroxide
Answers: 2

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1
You know the right answer?
The pH of a solution is a measure of hydrogen ion concentration, (H'). It can be calculated using th...
Questions

SAT, 11.07.2019 03:30

Mathematics, 11.07.2019 03:30



Chemistry, 11.07.2019 03:30

English, 11.07.2019 03:30

Chemistry, 11.07.2019 03:30



Arts, 11.07.2019 03:30



English, 11.07.2019 03:30

Biology, 11.07.2019 03:30


Biology, 11.07.2019 03:30


Mathematics, 11.07.2019 03:30


Computers and Technology, 11.07.2019 03:30



