Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
The name of the ion, s2-, is: sulfurous ion sulfide ion sulfur ion sulfate ion
Answers: 1

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1

Chemistry, 22.06.2019 20:20
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
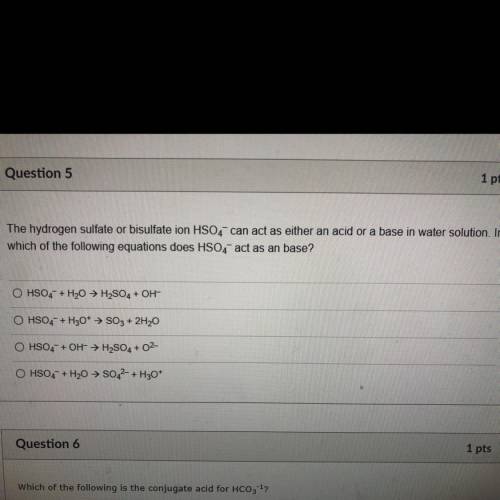
The hydrogen sulfate or bisulfate ion HSO4- can act as either an acid or a base in water solution. I...
Questions

English, 02.11.2020 01:40

Mathematics, 02.11.2020 01:40


Arts, 02.11.2020 01:40


Mathematics, 02.11.2020 01:40

English, 02.11.2020 01:40


Mathematics, 02.11.2020 01:40

Mathematics, 02.11.2020 01:40


Mathematics, 02.11.2020 01:40


Biology, 02.11.2020 01:40

Chemistry, 02.11.2020 01:40

Chemistry, 02.11.2020 01:40


History, 02.11.2020 01:40

Arts, 02.11.2020 01:40