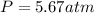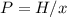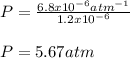Chemistry, 13.05.2021 23:10 mruffier6239
The mole fraction of helium in a saturated solution at 0°C is 1.2 x 10–6. Find the pressure of helium above the solution. Henry's law constant is 6.8 x 10–6 atm–1

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Esign techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 3

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1

Chemistry, 23.06.2019 07:00
0.88 moles of n2o5 (g) was placed in a sealed 1.00 l vessel. calculate the equilibrium concentration of n2o5. no2, and o2 and the equilibrium constant after equilibrium has been reached by 65.0% of the n2o5 decomposing.
Answers: 1

Chemistry, 23.06.2019 12:00
Jill is pushing a box across the floor. which represents the upward force perpendicular to the floor? a) fp b) ff c) fn d) fg
Answers: 1
You know the right answer?
The mole fraction of helium in a saturated solution at 0°C is 1.2 x 10–6. Find the pressure of heliu...
Questions






History, 01.08.2019 06:30

Social Studies, 01.08.2019 06:30

Social Studies, 01.08.2019 06:40

Mathematics, 01.08.2019 06:40

Social Studies, 01.08.2019 06:40

Computers and Technology, 01.08.2019 06:40

Mathematics, 01.08.2019 06:40




Physics, 01.08.2019 06:40




History, 01.08.2019 06:40