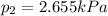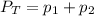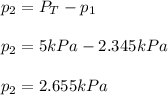Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3


You know the right answer?
The total pressure of two unknown gases is 5 kPa. The partial pressure of one of the gases is 2.345...
Questions


English, 23.07.2019 21:00

History, 23.07.2019 21:00




Arts, 23.07.2019 21:00

Biology, 23.07.2019 21:00


Biology, 23.07.2019 21:00

Mathematics, 23.07.2019 21:00

Social Studies, 23.07.2019 21:00

History, 23.07.2019 21:00


Mathematics, 23.07.2019 21:00



Mathematics, 23.07.2019 21:00

Social Studies, 23.07.2019 21:00