
The heat of combustion per mole for acetylene, C2H2(g), is -1299.5 kJ/mol. Assuming that the combustion products are CO2(g) and H2O(l), and given that the enthalpy of formation is -393.5 kJ/mol for CO2(g) and -285.8 kJ/mol for H2O(l), find the enthalpy of formation of C2H2(g).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
The heat of combustion per mole for acetylene, C2H2(g), is -1299.5 kJ/mol. Assuming that the combust...
Questions

Chemistry, 06.01.2021 14:00



Chemistry, 06.01.2021 14:00


Mathematics, 06.01.2021 14:00


Chemistry, 06.01.2021 14:00



Physics, 06.01.2021 14:00


Mathematics, 06.01.2021 14:00

Mathematics, 06.01.2021 14:00

Mathematics, 06.01.2021 14:00

Biology, 06.01.2021 14:00


History, 06.01.2021 14:00

Mathematics, 06.01.2021 14:00

Mathematics, 06.01.2021 14:00

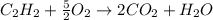
![\Delta H^{o}_{rxn} = \sum \Delta H_{products} - \sum \Delta H_{reactants}\\\Delta H^{o}_{rxn} = [2\Delta H^{o}_{f}(CO_{2}) + \Delta H^{o}_{f} (H_{2}O)] - [\Delta H^{o}_{f}(C_{2}H_{2}) + \frac{5}{2} \Delta H^{o}_{f} O_{2}]\\-1299.5 = 2(-393.5) + (-285.8) - \Delta H^{o}_{f} (C_{2}H_{2})\\\Delta H^{o}_{f} (C_{2}H_{2}) = 227.7 kJ/mol](/tpl/images/1323/2810/92eb3.png)


