
Chemistry, 14.05.2021 05:40 lescoto9035
Reaction 2:
10 Na, + 2 KNO
3 (s)
KO + 5 Nao +
2 (9)
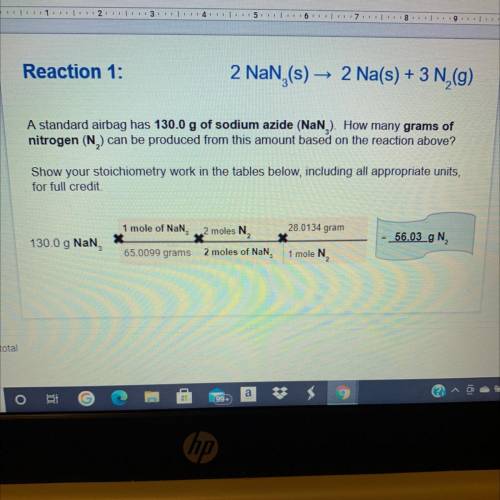
Based on the mass of sodium produced from reaction 1, how many of grams of
nitrogen will also be produced in reaction 2?
+
Show your stoichiometry work in the tables below, including all appropriate units,
for full credit
gN
46 g Na
This slide is worth 2.5 pts total

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 22:00
Which solution is an example of a nonelectrolyte? a. iodine in hexane b. sodium nitrate in waterc. acetic acid in waterd. hydrogen chloride in water
Answers: 2

Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1
You know the right answer?
Reaction 2:
10 Na, + 2 KNO
3 (s)
KO + 5 Nao +
2 (9)
Based on the mass of so...
3 (s)
KO + 5 Nao +
2 (9)
Based on the mass of so...
Questions


Mathematics, 27.02.2020 22:27




History, 27.02.2020 22:27

Mathematics, 27.02.2020 22:27





English, 27.02.2020 22:27



English, 27.02.2020 22:27

Chemistry, 27.02.2020 22:27


Mathematics, 27.02.2020 22:27

Physics, 27.02.2020 22:27



