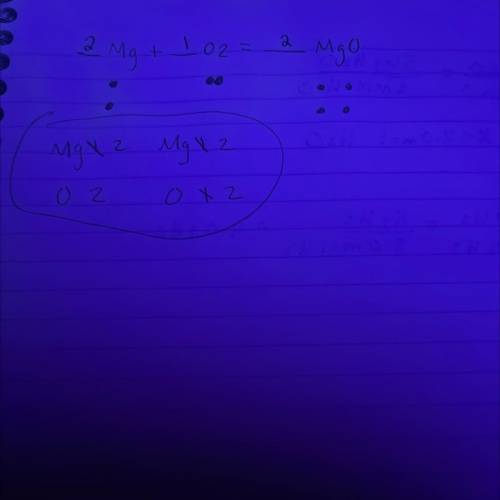In a balanced chemical reaction, the number of atoms of each element in the product(s) always equals the
A. molar mass of the reactants
B. proportional masses of the reactants
C. the number of atoms of each element in the reatants
D. the number of elements in the reactants

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3
You know the right answer?
In a balanced chemical reaction, the number of atoms of each element in the product(s) always equals...
Questions





Chemistry, 24.03.2021 18:40

History, 24.03.2021 18:40

Mathematics, 24.03.2021 18:40

Arts, 24.03.2021 18:40

Mathematics, 24.03.2021 18:40

Mathematics, 24.03.2021 18:40



Mathematics, 24.03.2021 18:40

French, 24.03.2021 18:40

Physics, 24.03.2021 18:40

Chemistry, 24.03.2021 18:40