
Chemistry, 14.05.2021 06:20 butterflyrhodes01
Considere la combustión del monóxido de carbono (CO) en oxígeno gaseoso: 2CO(g) + O2(g) → 2CO2(g) Si la reacción se inicia con 3.60 moles de CO, calcule el número de moles de CO2 que se producen si hay suficiente oxígeno para reaccionar con todo el CO.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2



Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
Considere la combustión del monóxido de carbono (CO) en oxígeno gaseoso: 2CO(g) + O2(g) → 2CO2(g) S...
Questions



Health, 20.05.2021 17:20

Mathematics, 20.05.2021 17:20




Chemistry, 20.05.2021 17:20




Biology, 20.05.2021 17:20

Mathematics, 20.05.2021 17:20

Mathematics, 20.05.2021 17:20

Mathematics, 20.05.2021 17:20



Mathematics, 20.05.2021 17:20

Mathematics, 20.05.2021 17:20

World Languages, 20.05.2021 17:20

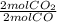 = 3.60 moles CO₂
= 3.60 moles CO₂

