
Chemistry, 14.05.2021 14:00 rubiim9610
What is the density of a gas at STP that has a molar mass of 50.0 g/mol?
Student A wrote some preliminary notes: PV = nRT; n = m/M; and d = m/V
known: unknown
T = 273 K d = g/cm3
P = 1 atm
R = 0.08125 atm
M = 50.0 g/mol
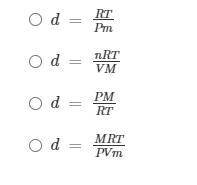
If PV = nRT and n = m/M, what is the density of the ideal gas?
Help Student A find the equation to use to solve this problem:

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1
You know the right answer?
What is the density of a gas at STP that has a molar mass of 50.0 g/mol?
Student A wrote some preli...
Questions

Mathematics, 21.01.2021 22:10

Computers and Technology, 21.01.2021 22:10

Arts, 21.01.2021 22:10

Business, 21.01.2021 22:10




Mathematics, 21.01.2021 22:10




Chemistry, 21.01.2021 22:10


Mathematics, 21.01.2021 22:10


Mathematics, 21.01.2021 22:10


Biology, 21.01.2021 22:10

Mathematics, 21.01.2021 22:10



