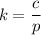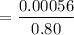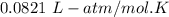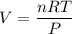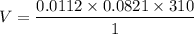The solubility of N2in blood at 37°C and at a partial pressure of 0.80 atm is 5.6 × 10−4mol/L. A deep-sea diver breathes compressed air with the partial pressure of N2equal to 4.0 atm. Assume that the total volume of blood in the body is 5.0 L. Calculate the amount of N2gas released (in liters at 37°C and 1 atm) when the diver returns to the surface of the water, where the partial pressure ofN2is 0.80 atm.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
The solubility of N2in blood at 37°C and at a partial pressure of 0.80 atm is 5.6 × 10−4mol/L. A dee...
Questions




English, 18.04.2020 00:04





Spanish, 18.04.2020 00:04










Mathematics, 18.04.2020 00:04