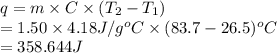Chemistry, 14.05.2021 18:40 shanicar33500
The specific heat capacity of liquid water is 4.18 J/g oC. Calculate the quantity of energy required to heat 1.50 g of water from 26.5oC to 83.7oC. (Ignore significant figures for this problem.)

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3
You know the right answer?
The specific heat capacity of liquid water is 4.18 J/g oC. Calculate the quantity of energy required...
Questions



History, 22.09.2020 01:01

English, 22.09.2020 01:01


Mathematics, 22.09.2020 01:01


English, 22.09.2020 01:01


Mathematics, 22.09.2020 01:01

Mathematics, 22.09.2020 01:01


Mathematics, 22.09.2020 01:01

Mathematics, 22.09.2020 01:01


Arts, 22.09.2020 01:01

Spanish, 22.09.2020 01:01

Mathematics, 22.09.2020 01:01


Mathematics, 22.09.2020 01:01

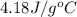
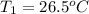
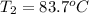
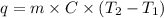
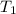 = initial temperature
= initial temperature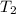 = final temperature
= final temperature