
Chemistry, 14.05.2021 21:50 Kittylover4812
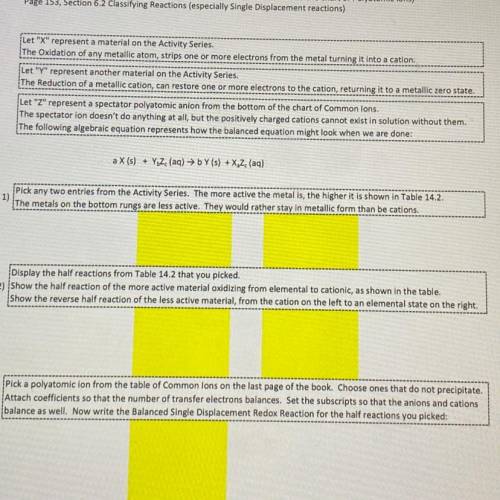
Let "X" represent a material on the Activity Series
The Oxidation of any metallic atom, strips one or more electrons from the metal turning it into a cation.
Let "Y" represent another material on the Activity Series.
The Reduction of a metallic cation, can restore one or more electrons to the cation, returning it to a metallic zero state.
Let "Z" represent a spectator polyatomic anion from the bottom of the chart of Common Ions.
The spectator ion doesn't do anything at all, but the positively charged cations cannot exist in solution without them.
The following algebraic equation represents how the balanced equation might look when we are done:
a X (s) + YZ (aq) → bY (s) + X. Z (aq)
1)
Pick any two entries from the Activity Series. The more active the metal is, the higher it is shown in Table 14.2.
The metals on the bottom rungs are less active. They would rather stay in metallic form than be cations
Display the half reactions from Table 14.2 that you picked
2) show the half reaction of the more active material oxidizing from elemental to cationic, as shown in the table.
show the reverse half reaction of the less active material, from the cation on the left to an elemental state on the right.
Pick a polyatomic ion from the table of Common ions on the last page of the book. Choose ones that do not precipitate.
3) Attach coefficients so that the number of transfer electrons balances. Set the subscripts so that the anions and cations
balance as well. Now write the Balanced Single Displacement Redox Reaction for the half reactions you picked:

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3


Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1
You know the right answer?
Let "X" represent a material on the Activity Series
The Oxidation of any metallic atom, strips one...
Questions



English, 05.12.2019 07:31


History, 05.12.2019 07:31

English, 05.12.2019 07:31

Social Studies, 05.12.2019 07:31

English, 05.12.2019 07:31


Mathematics, 05.12.2019 07:31

Business, 05.12.2019 07:31

Social Studies, 05.12.2019 07:31

English, 05.12.2019 07:31

Mathematics, 05.12.2019 07:31

Chemistry, 05.12.2019 07:31


Mathematics, 05.12.2019 07:31

Mathematics, 05.12.2019 07:31




