
Chemistry, 14.05.2021 23:20 saltyimps3
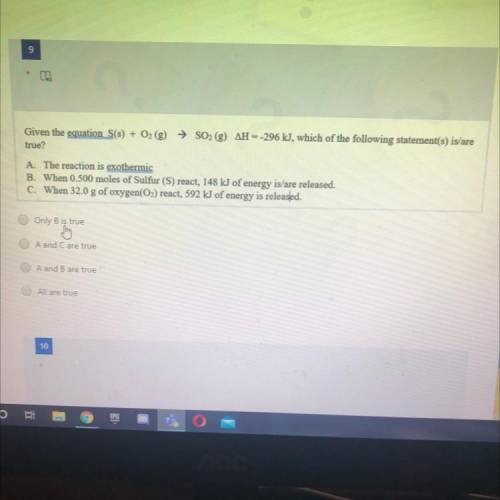
Given the equation S(s) + O2 (g) → SO2 (g) AH --296 kJ, which of the following statement(s) is/are
true?
A. The reaction is exothermic
B. When 0.500 moles of Sulfur (S) react, 148 kJ of energy is/are released.
C. When 32.0 g of oxygen(O2) react, 592 kJ of energy is released.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2
You know the right answer?
Given the equation S(s) + O2 (g) → SO2 (g) AH --296 kJ, which of the following statement(s) is/are...
Questions

Mathematics, 08.06.2021 19:20

Mathematics, 08.06.2021 19:20

History, 08.06.2021 19:20


Mathematics, 08.06.2021 19:20

Mathematics, 08.06.2021 19:20

Mathematics, 08.06.2021 19:20


Mathematics, 08.06.2021 19:20


Mathematics, 08.06.2021 19:20


Mathematics, 08.06.2021 19:20

History, 08.06.2021 19:20

Mathematics, 08.06.2021 19:20

Mathematics, 08.06.2021 19:20

Mathematics, 08.06.2021 19:20


English, 08.06.2021 19:20




