
Chemistry, 15.05.2021 03:10 tammycute01
He calculated that he should produced 6.0 grams of Mg2N2 . He actually produced 4.8 grams of Mg3N2 . What is the percent yield ?
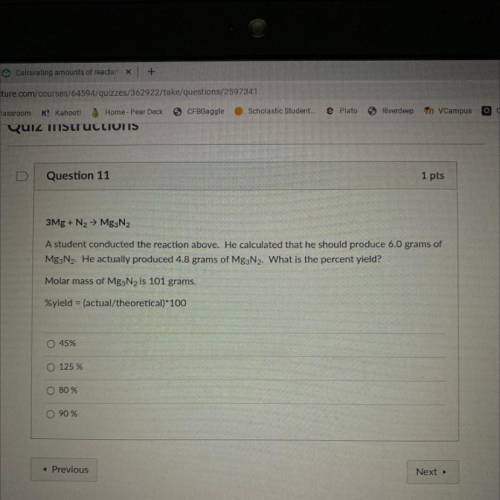

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which uses electromagnetic radiation to discover the properties and composition of bodies in space? space probe space station space shuttle space observatory
Answers: 2

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

You know the right answer?
He calculated that he should produced 6.0 grams of Mg2N2 . He actually produced 4.8 grams of Mg3N2 ....
Questions












History, 17.07.2019 07:00

Arts, 17.07.2019 07:00

Social Studies, 17.07.2019 07:00


English, 17.07.2019 07:00



Computers and Technology, 17.07.2019 07:00

History, 17.07.2019 07:00



