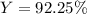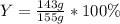Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Match term definition ellipse a) diagonal cross section of a cylinder circle b) diagonal cross section through the widest part of a sphere sphere c) cross section parallel to the base of a cone great circle d) shape created when a semi-circle is rotated around the y-axis triangle e) perpendicular cross section of a cone
Answers: 1


Chemistry, 22.06.2019 13:30
1) which of the following is the best example of a physical change? a) sugar dissolving in tea b) firefly glowing 2) in the combustion of ethane, what is/are the reactants? c2h6 + o2 ==> co2 + h2o a) c2h6 and o2 b) co2 and c2h6
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
For the reaction 2Na + Cl2 2NaCl, calculate the percent yield if
155.0 g of chlorine (Cl2) should b...
Questions



Biology, 20.05.2021 04:50


Physics, 20.05.2021 04:50

English, 20.05.2021 04:50

English, 20.05.2021 04:50

English, 20.05.2021 04:50

Physics, 20.05.2021 04:50


Mathematics, 20.05.2021 04:50

Health, 20.05.2021 04:50


Mathematics, 20.05.2021 04:50

Social Studies, 20.05.2021 04:50

Mathematics, 20.05.2021 04:50

Business, 20.05.2021 04:50


Chemistry, 20.05.2021 04:50