
Chemistry, 15.05.2021 05:10 ninaaforever
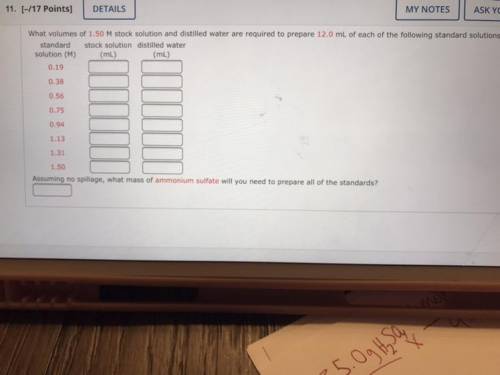
What volumes of 1.50 M stock solution and distilled water are required to prepare 12.0 mL of each of the following standard solutions:
standard stock solution distilled water
solution (M)
(mL)
0.19
(mL)
0.38
0.56
0.75
0.94
1.13
1.31
1.50
Assuming no spillage, what mass of ammonium sulfate will you need to prepare all of the standards?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 10:10
What shape would a molecule with two bound groups and two lone pairs have?
Answers: 1


Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1
You know the right answer?
What volumes of 1.50 M stock solution and distilled water are required to prepare 12.0 mL of each of...
Questions

Mathematics, 20.11.2020 01:00

History, 20.11.2020 01:00



Mathematics, 20.11.2020 01:00


Engineering, 20.11.2020 01:00

Biology, 20.11.2020 01:00

Physics, 20.11.2020 01:00

History, 20.11.2020 01:00



Physics, 20.11.2020 01:00

Biology, 20.11.2020 01:00


Mathematics, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

English, 20.11.2020 01:00

Mathematics, 20.11.2020 01:00

Chemistry, 20.11.2020 01:00



