
Chemistry, 15.05.2021 18:50 jasminebrown72
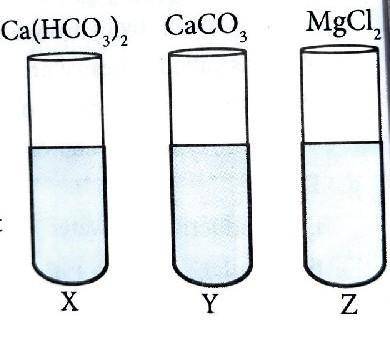
 X , Y and Z are the samples of water obtained from different sources. The types of salt dissolved in them are labelled in the picture. Now , Answer the following questions.
i. Identify the hardness of water that can be removed by boiling process.
X , Y and Z are the samples of water obtained from different sources. The types of salt dissolved in them are labelled in the picture. Now , Answer the following questions.
i. Identify the hardness of water that can be removed by boiling process.
ii. Which of them is soft water ?
iii. Write the chemical equation of the reaction that takes place when Z is treated with X , Y and Z.
~Random/Irrelevant answers will be reported!

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 19:00
Which change to the system wood cause the freely-moving piston to lower?
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3 2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1
You know the right answer?
X , Y and Z are the samples of water obtained from different sources. The types of salt dissolved i...
Questions

Mathematics, 18.08.2019 22:50

History, 18.08.2019 22:50

Mathematics, 18.08.2019 22:50

Mathematics, 18.08.2019 22:50

Biology, 18.08.2019 22:50

Chemistry, 18.08.2019 22:50


History, 18.08.2019 22:50



Mathematics, 18.08.2019 22:50

Geography, 18.08.2019 22:50


English, 18.08.2019 22:50

Biology, 18.08.2019 22:50

Biology, 18.08.2019 22:50

Social Studies, 18.08.2019 22:50


Biology, 18.08.2019 22:50








