
Chemistry, 16.05.2021 06:10 Alexhall112
A helium-filled balloon has a volume of 50.0 L at 1.08 atm. What volume will it have at 0.588 atm and 10.0 C?
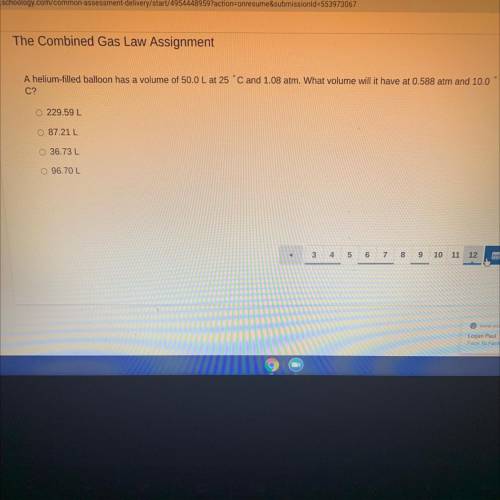

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3


Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
You know the right answer?
A helium-filled balloon has a volume of 50.0 L at 1.08 atm. What volume will it have at 0.588 atm an...
Questions

English, 02.11.2020 07:20

Mathematics, 02.11.2020 07:20

English, 02.11.2020 07:20



Mathematics, 02.11.2020 07:20

Mathematics, 02.11.2020 07:20


Mathematics, 02.11.2020 07:20

Arts, 02.11.2020 07:20


Mathematics, 02.11.2020 07:20

Spanish, 02.11.2020 07:20

Mathematics, 02.11.2020 07:20

History, 02.11.2020 07:20



Mathematics, 02.11.2020 07:20





