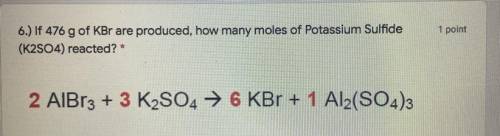
6.) If 476 g of KBr are produced, how many moles of Potassium Sulfide
(K2SO4) reacted? *
2 Al...


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

You know the right answer?
Questions

Mathematics, 28.01.2020 04:31

Physics, 28.01.2020 04:31


Mathematics, 28.01.2020 04:31

Mathematics, 28.01.2020 04:31

Social Studies, 28.01.2020 04:31



Mathematics, 28.01.2020 04:31



Social Studies, 28.01.2020 04:31


Social Studies, 28.01.2020 04:31

Health, 28.01.2020 04:31


Chemistry, 28.01.2020 04:31


Mathematics, 28.01.2020 04:31

Computers and Technology, 28.01.2020 04:31