

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3


Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1
You know the right answer?
a sample of sulfur contains sulfur-32, sulfur-33, sulfur-34, and sulfur-36 atoms. why these atoms ca...
Questions


Chemistry, 14.07.2019 07:30



Mathematics, 14.07.2019 07:30

Mathematics, 14.07.2019 07:30



Spanish, 14.07.2019 07:30

Chemistry, 14.07.2019 07:30







Chemistry, 14.07.2019 07:30

Social Studies, 14.07.2019 07:30


Mathematics, 14.07.2019 07:30

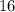 .) Hence, the atomic number of each atom would be equal to the atomic number of sulfur:
.) Hence, the atomic number of each atom would be equal to the atomic number of sulfur: 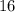 .
. 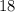 belongs to argon.
belongs to argon.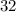 (mass number
(mass number 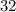 ) contains
) contains 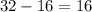 neutrons, whereas sulfur-
neutrons, whereas sulfur-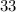 (mass number
(mass number 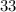 ) contains
) contains 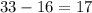 neutrons. (The
neutrons. (The 

