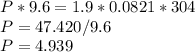Chemistry, 17.05.2021 17:40 loveyeti106838
At 304 K, a 9.6 L tank contains 1.9 moles of H2 gas under an unknown pressure in atm. What is the pressure of the gas in the tank? (R = 0.0821 L*atm/mol*K)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3


Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
You know the right answer?
At 304 K, a 9.6 L tank contains 1.9 moles of H2 gas under an unknown pressure in atm. What is the pr...
Questions


Mathematics, 01.04.2020 00:20

Physics, 01.04.2020 00:20






Chemistry, 01.04.2020 00:20

Mathematics, 01.04.2020 00:20




Mathematics, 01.04.2020 00:20

History, 01.04.2020 00:20

Mathematics, 01.04.2020 00:20

English, 01.04.2020 00:20

Mathematics, 01.04.2020 00:20