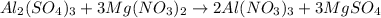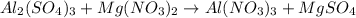Chemistry, 17.05.2021 17:40 sofiisabella10
Al2(SO4)3+Mg(NO3)2⟶ What would be the product(s) of this reaction? *These are NOT balanced, just look for the correct products*

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 01:00
Atoms contain subatomic particles called protons and neutrons. when these protons and neutrons spilt, a lot of energy is released
Answers: 3

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

Chemistry, 23.06.2019 15:50
Many radioactive atoms that have large masses undergo radioactive decay by releasing a particle that is identical to a helium-4 nucleus. what changes in the original atom are expected as a result of this natural phenomenon? the atomic number and the mass number will decrease. the atomic number and the mass number will increase. the atomic number will increase, and the mass number will decrease. the atomic number will decrease, and the mass number will increase.
Answers: 2
You know the right answer?
Al2(SO4)3+Mg(NO3)2⟶ What would be the product(s) of this reaction? *These are NOT balanced, just loo...
Questions


Geography, 05.05.2021 21:00


Computers and Technology, 05.05.2021 21:00




Social Studies, 05.05.2021 21:00

Mathematics, 05.05.2021 21:00




History, 05.05.2021 21:00




Mathematics, 05.05.2021 21:00



Mathematics, 05.05.2021 21:00