Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 08:30
Which metal exist in liquid state and can be cut with knife ?
Answers: 2

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
Pls help 20 points
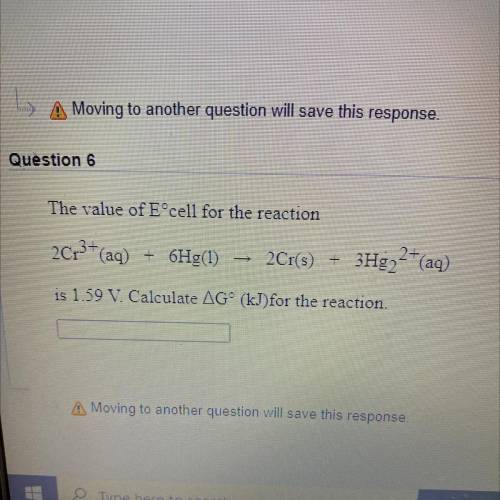
Question 6
The value of Eºcell for the reaction
2013+ (aq) + 6Hg(1)<...
The value of Eºcell for the reaction
2013+ (aq) + 6Hg(1)<...
Questions

Mathematics, 21.05.2020 04:09



Mathematics, 21.05.2020 04:09



Mathematics, 21.05.2020 04:09


Chemistry, 21.05.2020 04:09


English, 21.05.2020 04:09


History, 21.05.2020 04:09


Mathematics, 21.05.2020 04:09



Mathematics, 21.05.2020 04:09


History, 21.05.2020 04:09