A. Keg


Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
You know the right answer?
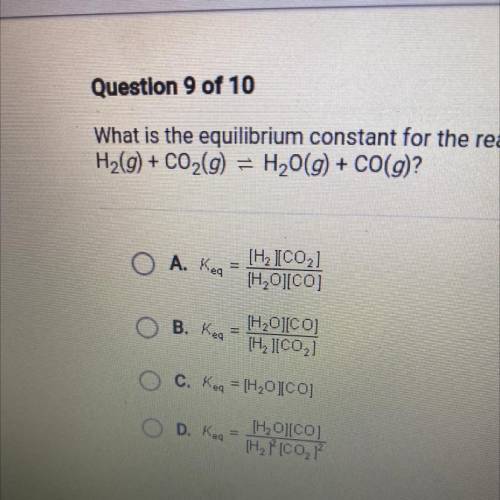
What is the equilibrium constant for the reaction
H2(g) + CO2(g) = H2O(g) + CO(g)?
A. Keg
A. Keg
Questions


Biology, 03.04.2021 21:00


History, 03.04.2021 21:00



English, 03.04.2021 21:00

Chemistry, 03.04.2021 21:00



Mathematics, 03.04.2021 21:00

Social Studies, 03.04.2021 21:00

Mathematics, 03.04.2021 21:00



Mathematics, 03.04.2021 21:00



Mathematics, 03.04.2021 21:00