On 2
6 pts
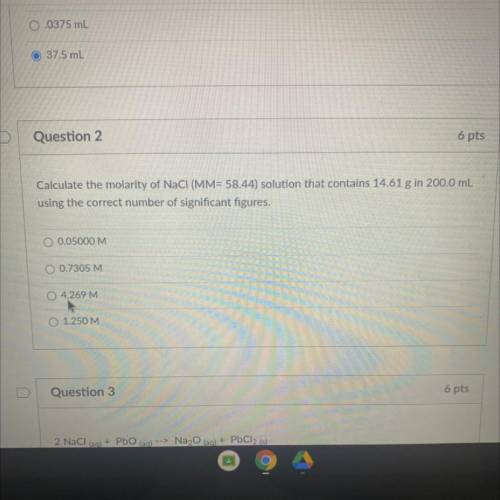
Calculate the molarity of NaCl (MM= 58.44) solution that contains 14.61 g in 200,0...

Chemistry, 17.05.2021 22:00 xeskimopie
On 2
6 pts
Calculate the molarity of NaCl (MM= 58.44) solution that contains 14.61 g in 200,0 mL
using the correct number of significant figures,
O 0.05000 M
O 0.7305 M
O 4.269 M
O 1.250 M

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2


Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Questions

Geography, 01.10.2021 22:40

Mathematics, 01.10.2021 22:40

Mathematics, 01.10.2021 22:40



Physics, 01.10.2021 22:40





Mathematics, 01.10.2021 22:40

Mathematics, 01.10.2021 22:40


Mathematics, 01.10.2021 22:40


Advanced Placement (AP), 01.10.2021 22:40


Mathematics, 01.10.2021 22:40


Social Studies, 01.10.2021 22:40



