
Chemistry, 18.05.2021 06:00 ineedhelpplz40
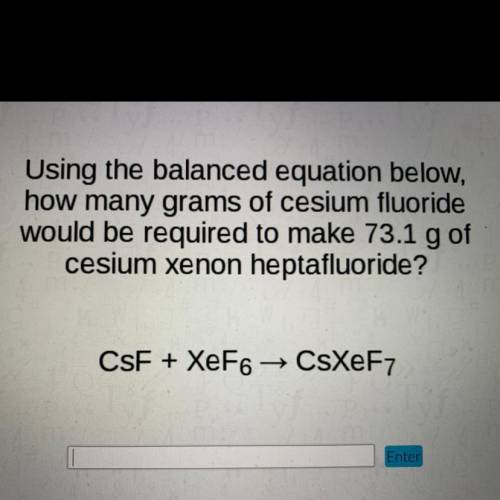
Using the balanced equation below, how many grams of cesium fluoride would be required to make 73.1 g of cesium xenon heptafluoride? CSF + XeF6 → CsXeF7

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 23.06.2019 05:00
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius.a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit.a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius.a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
You know the right answer?
Using the balanced equation below, how many grams of cesium fluoride would be required to make 73.1...
Questions


Computers and Technology, 31.05.2021 21:50

Mathematics, 31.05.2021 21:50



Social Studies, 31.05.2021 21:50

Mathematics, 31.05.2021 21:50

Mathematics, 31.05.2021 21:50

Health, 31.05.2021 21:50

Mathematics, 31.05.2021 21:50


History, 31.05.2021 21:50







Mathematics, 31.05.2021 21:50

Biology, 31.05.2021 21:50



