
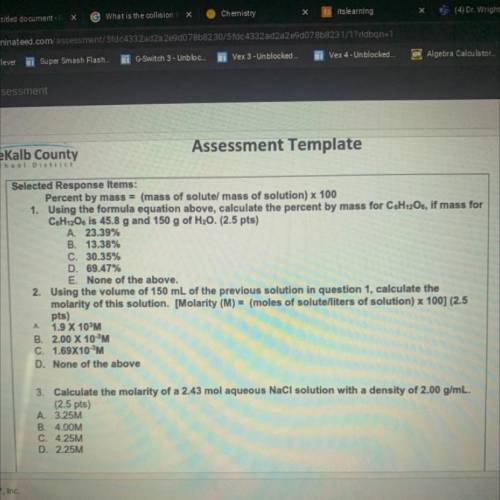
Chemistry, 18.05.2021 17:50 Tonyang1742
Percent by mass = (mass of solutel mass of solution) x 100
1. Using the formula equation above, calculate the percent by mass for C&H12O6, if mass for
C6H12Os is 45.8 g and 150 g of H20. (2.5 pts)
A. 23.39%
B. 13.38%
C. 30.35%
D. 69.47%
E. None of the above.
2. Using the volume of 150 mL of the previous solution in question 1, calculate the
molarity of this solution. [Molarity (M) = (moles of solute/liters of solution) x 100] (2.5
pts)
1.9 X 10%M
B. 2.00 X 10 M
C. 1.69X10M
D. None of the above
A
3. Calculate the molarity of a 2.43 mol aqueous NaCl solution with a density of 2.00 g/mL.
(2.5 pts)
A 3.25M
B. 4.00M
C. 4.25M
D. 2.25M

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 22.06.2019 13:30
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
Percent by mass = (mass of solutel mass of solution) x 100
1. Using the formula equation above, cal...
Questions






Mathematics, 20.07.2019 03:00

Mathematics, 20.07.2019 03:00

Spanish, 20.07.2019 03:00

Health, 20.07.2019 03:00


Mathematics, 20.07.2019 03:00






Spanish, 20.07.2019 03:00


Social Studies, 20.07.2019 03:00

Health, 20.07.2019 03:00



