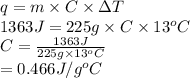Chemistry, 18.05.2021 18:30 golderhadashaowtatz
Calculate the specific heat capacity of the unknown metal given the
following information; the mass of the metal is 225 g, the temperature
raises by 13C and the energy absorbed was 1363 joules.
A. 0.466 j/gC
B.1.02j/gC
C.0.96 j/gC
D.2.14 j/gC

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2

Chemistry, 22.06.2019 22:00
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a.rectant b.product c.supernate
Answers: 3

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1
You know the right answer?
Calculate the specific heat capacity of the unknown metal given the
following information; the mass...
Questions



Mathematics, 21.12.2020 01:00

Business, 21.12.2020 01:00

Mathematics, 21.12.2020 01:00

History, 21.12.2020 01:00


Biology, 21.12.2020 01:00





Mathematics, 21.12.2020 01:00

Mathematics, 21.12.2020 01:00

Social Studies, 21.12.2020 01:00


Mathematics, 21.12.2020 01:00

Physics, 21.12.2020 01:00


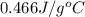 .
.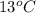
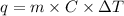
 = change in temperature
= change in temperature