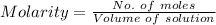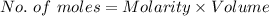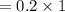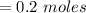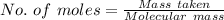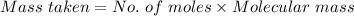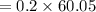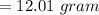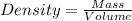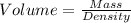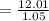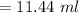Chemistry, 18.05.2021 19:10 phancharamachasm
You must make 1 L of 0.2 M acetic acid (CH3COOH). All you have available is concentrated glacial acetic acid (assay value, 98%; specific gravity, 1.05 g/mL). It will take milliliters of acetic acid to make this solution. Assume a gram molecular weight of 60.05 grams.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 22:30
Which of the following is not an assumption that scientists must make about the natural world? a. regularity b. causality c. predictability d. plausibility
Answers: 1
You know the right answer?
You must make 1 L of 0.2 M acetic acid (CH3COOH). All you have available is concentrated glacial ace...
Questions

History, 05.10.2019 23:40


Biology, 05.10.2019 23:40






Mathematics, 05.10.2019 23:40



Social Studies, 05.10.2019 23:50



English, 05.10.2019 23:50

Mathematics, 05.10.2019 23:50


History, 05.10.2019 23:50

Geography, 05.10.2019 23:50